Recently, the United States Department of Health and Human Services Office of Inspector General (HHS OIG) completed a review of the Open Payments data for accuracy, precision, and consistency in reporting.
OIG was prompted to perform this review because the transparency of the Open Payments program not only reveals the nature and extent of relationships between physicians and teaching hospitals with applicable manufacturers and group purchasing organizations, but also has the potential to discourage the development of inappropriate financial relationships. As noted in the report, the public can only benefit from the information if it is complete and accurate.
To determine the extent to which data reported were missing elements, or were inaccurate or inconsistent, HHS OIG downloaded data for 2015 from the Open Payments website in June of 2016, relatively soon after the data were collected. Then, to determine the role of CMS in validating Open Payments data received from manufacturers and group purchasing organizations (GPOs), the group reviewed policies and procedures and other information that CMS provided regarding its oversight.
Results from the Review
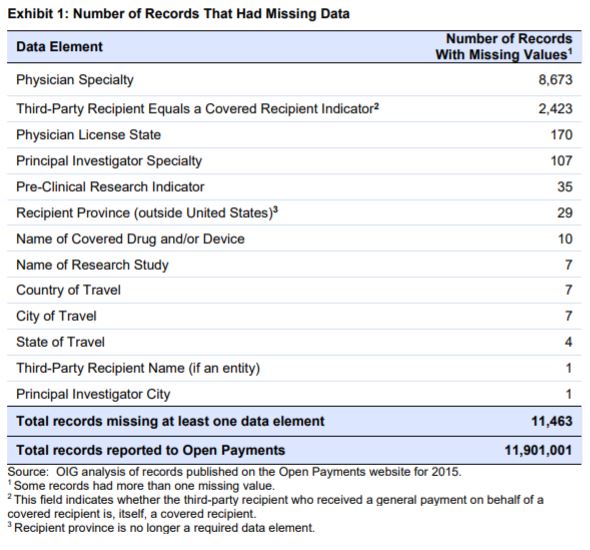
Of 11.9 million records published on the Open Payments website for 2015, less than 1 percent (0.09%) were missing required data elements. Although the Open Payments data elements reported to CMS were complete overall, we did identify records that contained inaccurate, imprecise, or inconsistent information. These include records containing drug and device names that do not match the definitions of these data elements; national drug codes (NDCs) that were not found in multiple Food and Drug Administration databases or other drug information resources; and payment dates from a different reporting year. CMS did note that it has conducted outreach to address data concerns with manufacturers and group purchasing organizations. CMS also reported that it is still compiling a list of noncompliant manufacturers and group purchasing organizations for further investigation.
Further, even though the Open Payments system validates whether the payment date is in the correct submission format and whether the year reported is the same as the program year, there were 565 records that had a payment date that was either earlier or later than 2015. According to CMS, manufacturers and GPOs that submitted records with dates outside program year 2015 were notified by phone and a followup email and were asked to correct the records, resubmit them, and provide re-attestations. Those corrected records were not reflected in the June 2016 publication of the data, and are therefore not included in this review.
It should also be noted that OIG did not determine whether there were manufacturers and GPOs that failed to submit all required financial relationships for all covered recipients.
In-Depth Review
Most of the records found contained the required fields 99.9% of the time. This means that much less than 1% of missing records had missing data elements – 11,463 records out of 11.9 million records. Further, a vast majority of the 0.09% missing record values were for fields such as physician specialty (75%) or name of the third party who received the payment (21%).
CMS is expected to be developing audit strategies and plans, but has not yet conducted any audits of manufacturers or GPOs.
It is also important to remember that in 2015, there were problems submitting data through Open Payments because of CMS’ name matching protocol, but that issue has since been resolved.
HHS OIG Recommendations
At the conclusion of the review, HHS OIG provides four recommendations to improve the accuracy, precision, and consistency of the data to help consumers better use the information: (1) ensure that records contain all required data; (2) strengthen validation rules and revise data-element definitions so that actual drug and device names must be reported; (3) revise the definition of the device-name data element so that the information reported is required to be more specific; and (4) ensure that manufacturers and group purchasing organizations report valid NDCs for drugs. According to HHS OIG, CMS concurred with all four of our recommendations.